Introduction
Bone is a mineralized connective tissue that exhibits four types of cells: osteoclasts, bone lining cells, osteocytes, and osteoclasts. Bone exerts important functions in the body, such as locomotion, support, and protection of soft tissues, calcium and phosphate storage, and harboring of bone marrow. Despite its inert appearance, bone is a highly dynamic organ that is continuously reabsorbed by osteoclasts and reformed by osteoblasts. There is evidence that osteocytes act as mechanosensors and orchestrators of this bone remodeling process. This is introduction to the histology of the bone.
The function of bone lining cells is not well clear, but these cells seem to play an important role in coupling bone resorption to bone formation. Bone remodeling is a highly complex process by which old bone is replaced by new bone, in a cycle comprised of three phases:
- Initiation of bone resorption by osteoclasts,
- The transition (or reversal period) from resorption to new bone formation, and
- The bone formation by osteoblasts.
This process occurs due to coordinated actions of osteoclasts, osteoblasts, osteocytes, and bone lining cells which together, form the temporary anatomical structure called basic multicellular unit (BMU). Normal bone remodeling is necessary for fracture healing and skeleton adaptation to mechanical use, as well as for calcium homeostasis.
On the other hand, an imbalance of bone resorption and formation results in several bone diseases. For example, excessive resorption by osteoclasts without the corresponding amount of nerformed bone by osteoblasts contributes to bone loss and osteoporosis, whereas the contrary may result in osteopetrosis.
Recent studies have shown that bone influences the activity of other organs and the bone is also influenced by other organs and systems of the body, providing new insights and evidencing the complexity and dynamic nature of bone tissue. In this review we will address the current data about bone cells biology, bone matrix, and the factors that influence the bone remodeling process. Moreover, we will briefly discuss the role of estrogen on bone tissue under physiological and pathological conditions.
Bone Cells
Osteoblasts
Osteoblasts are cuboidal cells that are located along the bone surface comprising 4–6% of the total resident bone cells and are largely known for their bone-forming function. These cells show morphological characteristics of protein-synthesizing cells, including abundant rough endoplasmic reticulum and prominent Golgi apparatus, as well as various secretory vesicles. As polarized cells, the osteoblasts secrete the osteoid toward the bone matrix. Osteoblasts are derived from mesenchymal stem cells (MSC).
The commitment of MSC towards the osteoprogenitor lineage requires the expression of specific genes, following timely programmed steps, including the synthesis of bone morphogenetic proteins (BMPs) and members of the Wingless (Wnt) pathways. The expressions of Runt related transcription factors 2, Distal-less home box 5 (Dlx5), and osterix (Osx) are crucial for osteoblast differentiation.
Additionally, Runx2 is a master gene of osteoblast differentiation, as demonstrated by the fact that Runx2-null mice are devoid of osteoblasts. Runx2 has demonstrated to upregulate osteoblast-related genes such as ColIA1, ALP, BSP, BGLAP, and OCN. Once a pool of osteoblast progenitors expressing Runx2and ColIA1 has been established during osteoblast differentiation, there is a proliferation phase.
[grid_creator id = ‘5’]In this phase, osteoblast progenitors show alkaline phosphatase (ALP) activity and are considered preosteoblasts. There is evidence that other factors such as fibroblast growth factor (FGF), microRNAs, and connexin 43 play important roles in osteoblast differentiation. FGF-2knockoutmice showed a decreased bone mass coupled to an increase of adipocytes in the bone marrow, indicating the participation of FGFs in the osteoblast differentiation. It has also been demonstrated that FGF-18 upregulates osteoblast differentiation in an autocrine mechanism.
MicroRNAs are involved in the regulation of gene expression in many cell types, including osteoblasts, in which some microRNAs stimulate and others inhibit osteoblast differentiation. Connexin 43 is known to be the main connexin in bone. The mutation in the gene encoding connexin 43 impairs osteoblast differentiation and causes skeletal malformation in mice. The synthesis of bone matrix by osteoblasts occurs in two main steps: deposition of organic matrix and its subsequent mineralization.
In the first step, the osteoblasts secrete collagen proteins, mainly type I collagen, noncollagenous proteins (OCN, osteonectin, BSP II, and osteopontin), and proteoglycan including decorin and biglycan, which form the organic matrix. Thereafter, mineralization of bone matrix takes place into two phases: the vesicular and the fibrillar phases. The vesicular phase occurs when portions with a variable diameter ranging from 30 to 200 nm, called matrix vesicles, are released from the apical membrane domain of the osteoblasts into the newly formed bone matrix in which they bind to proteoglycans and other organic components.
[grid_creator id = ‘2’]Because of its negative charge, the sulfated proteoglycans immobilize calcium ions that are stored within the matrix vesicles. When osteoblasts secrete enzymes that degrade the proteoglycans, the calcium ions are released from the proteoglycans and cross the calcium channels presented in the matrix vesicle membrane. These channels are formed by proteins called annexins. On the other hand, phosphate-containing compounds are degraded by the ALP secreted by osteoblasts, releasing phosphate ions inside the matrix vesicles. Then, the phosphate and calcium ions inside the vesicles nucleate, forming the hydroxyapatite crystals.
The fibrillar phase occurs when the supersaturation of calcium and phosphate ions inside the matrix vesicles leads to the rupture of these structures and the hydroxyapatite crystals spread to the surrounding matrix. Mature osteoblasts appear as a single layer of cuboidal cells containing abundant rough endoplasmic reticulum and large Golgi complex. Some of these osteoblasts show cytoplasmic processes towards the bone matrix and reach the osteocyte processes. At this stage, the mature osteoblasts can undergo apoptosis or become osteocytes or bone lining cells.
Interestingly, round/ovoid structures containing dense bodies and TUNEL-positive structures have been observed inside osteoblast vacuoles. These findings suggest that besides professional phagocytes, osteoblasts are also able to engulf and degrade apoptotic bodies during alveolar bone formation. Bone Lining Cells. Bone lining cells are quiescent flat-shaped osteoblasts that cover the bone surfaces, where neither bone resorption nor bone formation occurs.
[grid_creator id = ‘5’]These cells exhibit a thin and flat nuclear profile; its cytoplasm extends along the bone surface and displays few cytoplasmic organelles such as profiles of rough endoplasmic reticulum and Golgi apparatus. Some of these cells show processes extending into canaliculi, and gap junctions are also observed between adjacent bone lining cells and between these cells and osteocytes.
The secretory activity of bone lining cells depends on the bone physiological status, whereby these cells can reacquire their secretory activity, enhancing their size, and adopting a cuboidal appearance. Bone lining cells functions are not completely understood, but it has been shown that these cells prevent the direct interaction between osteoclasts and bone matrix, when bone resorption should not occur, and also participate in osteoclast differentiation, producing osteoprotegerin (OPG) and the receptor activator of nuclear factor kappa-B ligand (RANKL).
Moreover, the bone lining cells, together with other bone cells, are an important component of the BMU, an anatomical structure that is present during the bone remodeling cycle.
Types of Cartilage
- Hyaline Cartilage:
- Elastic cartilage
- Fibrocartilage
Hyaline Cartilage
They have identical form, covered by Perichondrium. They are Matrix formed by the Chondrocyte, the matrix is subdivided into two types:- Territorial and Interterritorial.
This is similar to hyaline cartilage. The matrix contain more elastic fibers as well as elastic fibers as well as type II collagen fibers.
Fibro-cartilage
This is different from Hyaline or Elastic. The chondrocytes are differentiated from fibrocytes.
Cartilage Growth
Cartilage grows by two different processes: Interstitial growth and Appositional growth
a. Interstitial growth: This involves the division of existing chondrocytes. It is important in the formation of the fetal skeleton and continues in the epiphyseal plates and articular cartilages.
b. Appositional growth: This involves the differentiation of chondroblasts and stem cells on the inner surface of the perichondrium into chondrocytes. It is responsible for increasing the girth of the cartilage masses.
[grid_creator id = ‘5’]Bone
The Bone tissue is rich in blood supply. It is a type of connective tissue with calcified matrix.
Functions
- It supports and protects
- Has Bone marrow.
- It is an important storage site for calcium and other essential minerals.
Surfaces
- The outer surfaces
- Sharpey’s fibers
- The internal surfaces of bones are covered by a thin, condensed reticular connective tissue (endosteum) that contain bone (endosteum) that contains bone and blood, cell precursors.
- The endosteum lines the marrow cavity and sends extensions into the haversian canals.
Components of Bone:
Osteogeneic cells, Osteoblasts, Osteocytes, Osteoclasts. Organic and non-organic collagen type I fibers.
Osteogenic (Osteoprogenitor) cells
- Mesenchymal stem cells, Small spindle-shaped cells with pale cytoplasm and ovoid nuclei.
- Osteoblast precursors have sparse RER and Golgi complexes.
- Osteoclast precursors have abundant free ribosomes and mitochondria.
Osteoblasts:
Found in the periosteum and endosteum. They have large rounded or cuboidal cells, with deep basophilic cytoplasm, with deep basophilic cytoplasm, well-developed RER and Golgi; and eccentric nucleus. They synthesize and secrete organic components. Osteoblasts mature and are called osteocytes.
Osteocytes
Theirs Cells are branched, smaller than osteoblasts, and not divide. They found in cavities in the bone matrix called lacunae. Processes (branches) extend into canaliculi in the calcified matrix. The cells are isolated. They Contact one another through gap junctions. Located near bone surfaces. They maintain bone matrix. The death of osteocytes results in bone resorption.
Osteoclasts
They lie on bony surfaces in Hawship’s lacunae. Have Large multinucleated cells. Acidophilic, cytoplasm containing abundant lysosomes and Acidophilic cytoplasm containing abundant lysosomes and mitochondria and well developed Golgi complex; and brush border of plasma-membrane facing the bone marrow. Cells release acid, collagenase, and in other lytic enzymes into the compartments; these break down bone matrix and release minerals, a process called bone resorption, Ossification (Bone formation). The bone is formed from mesenchymal embryonic tissue by two ways:
- Intra-membranous ossification
- Endochondral ossification
Intra-Membranous Ossification:
Ossification center in the form of increased vascularity, condensation of mesenchymal cells. The mesenchymal cells osteogenic cells. The osteogenic cells differentiate into osteoblasts; which synthesize the organic component of bone matrix (collagen, glycoprotein). The osteoblasts secrete an enzyme which stimulates calcium salts to the calcified matrix. The bone matrix grows in the membrane, vascular tissues differentiate into hematopoietic tissue (bone marrow). A layer of vascular mesenchyme forming periosteum. The osteogenic stem cells in the periosteum differentiate to osteoblasts, which start to lay down or arrange bone lamellae (compact bone).
Endochondral Ossification:
- Cartilage model: The embryonic mesenchymal cells -condensed. It is differentiated from chondroblasts, chondrocytes, and cartilage matrix.
- Primary centers of ossification: Some changes have been developed. The Chondrocytes in the middle of the cartilage model enlarged, and calcium salts become to deposit around the lacunae. Chondrocytes die due to the prevention of nutrient diffusion through the calcified matrix, leaving empty spaces.
- The perichondrium becomes highly vascular and active and changed to the periosteum, where the inner vascular osteogenic cells differentiate to osteoblasts.
- The osteoblasts start to lay down a collar of compact bone around the shaft called sub-periosteal collar of bone.
- The periosteum forms a periosteal bud, which is consists of capillaries, osteoclasts, and osteoblasts.
- The vascular bud invades the subperiosteal bone by osteoclasts.
- The thin wall of the empty lacunae is broken down.
- The thin wall of the empty lacunae is broken down forming the primary bone marrow cavity.
- As the periosteal bud invades bone and cartilage, the osteoblasts arrange themselves along the marrow spaces and start to lay down bone matrix
- Marrow spaces joint together forming a central regular cavity in the middle of diaphysis.
- The microscopic structure of the developing endochondral bones is characterized by 5 overlapping zones:
- The zone of resting cartilage is composed of typical hyaline cartilage and is farthest from the primary marrow cavity.
- The zone of proliferation contains columns of flattened chondrocytes.
- In the zone of hypertrophy, the chondrocytes in the columns are enlarged and rounded.
- The zone of calcification is characterized by a more basophilic matrix. There is often a significant overlap between zones 3 and 4, which are sometimes referred to as a single zone of hypertrophy and calcification.
- The zone of ossification borders directly on the primary marrow cavity. It is characterized by intensely acidophilic osteoid, osteocytes within the bone matrix, and a monolayer of basophilic osteoblasts.
Bone Functions
- Support
- Protection (protect internal organs)
- Movement (provide leverage system for skeletal muscles, tendons, ligaments and joints)
- Mineral homeostasis (bones act as reserves of minerals important for the body like calcium or phosphorus)
- Hematopoiesis: blood cell formation
- Storage of adipose tissue: yellow marrow
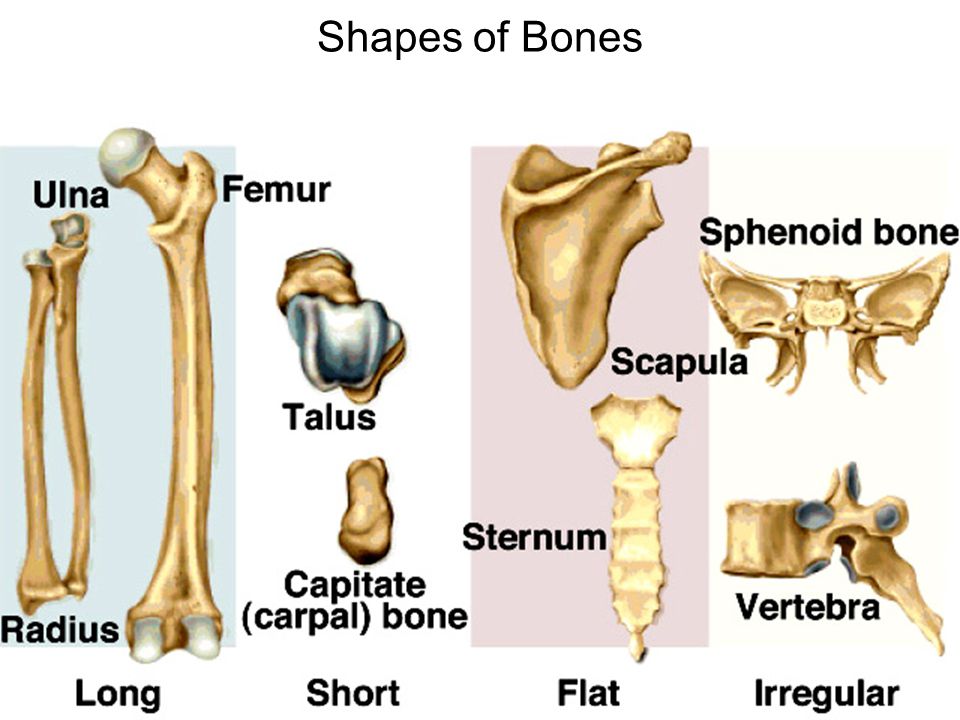
General Features of Bones
- Shaft (diaphysis) – cylinder of compact bone–marrow cavity (medullary cavity) lined with endosteum (osteogenic cells and reticular connective tissue)
- Enlarged ends (epiphyses) – spongy bone covered by compact bone enlarged to strengthen joint and attach ligaments
- Joint surface covered with articular cartilage (hyaline).
- Shaft covered with periosteum
- Outer fibrous layer of collagen
- Inner osteogenic layer of bone forming cells
- Endosteum membrane lining central canals and perforating canals.
- Epiphyseal plate (growth plate)
Compact Bone
- Osteon – basic structural unit cylinders formed from layers (lamellae) of matrix around central canal (osteonic canal)
- Collagen fibers alternate between right- and left-handed helices from lamella to lamella
- Osteocytes connected to each other and their blood supply by tiny cell processes in canaliculi
- Perforating canals or Volkmann canals
- vascular canals perpendicularly joining central canals
Healing of Fractures
Normally 8 – 12 weeks (longer in elderly)
References
A. Buckwalter, M. J. Glimcher, R. R. Cooper, and R. Recker, “Bone biology. I: structure, blood supply, cells, matrix, and mineralization,” Instructional Course Lectures, vol. 45, pp. 371–386, 1996.
P. A. Downey and M. I. Siegel, “Bone biology and the clinical implications for osteoporosis,” Physical Therapy, vol. 86, no. 1, pp. 77–91, 2006.
A. G. Robling, A. B. Castillo, and C. H. Turner, “Biomechanical and molecular regulation of bone remodeling,” Annual Review of Biomedical Engineering, vol. 8, pp. 455–498, 2006.
H. K. Datta, W. F. Ng, J. A. Walker, S. P. Tuck, and S. S. Varanasi, “The cell biology of bone metabolism,” Journal of Clinical Pathology, vol. 61, no. 5, pp. 577–587, 2008.
B. Clarke, “Normal bone anatomy and physiology,” Clinical Journal of the American Society of Nephrology, vol. 3, no. 3, pp. 131–139, 2008.
G. Karsenty, H. M. Kronenberg, and C. Settembre, “Genetic control of bone formation,” Annual Review of Cell and Developmental Biology, vol. 25, pp. 629–648, 2009.
S. L. Teitelbaum, “Osteoclasts: what do they do and how do they do it?” The American Journal of Pathology, vol. 170, no. 2, pp. 427–435, 2007.
L. F. Bonewald, “The amazing osteocyte,” Journal of Bone and Mineral Research, vol. 26, no. 2, pp. 229–238, 2011.
V. Everts, J. M. Delaissi´e, W. Korper et al., “The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation,” Journal of Bone and Mineral Research, vol. 17, no. 1, pp. 77–90, 2002.
N. A. Sims and J. H. Gooi, “Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption,” Seminars in Cell and Developmental Biology, vol. 19, no. 5, pp. 444–451, 2008. [11] K. Matsuo and N. Irie, “Osteoclast-osteoblast communication,” Archives of Biochemistry and Biophysics, vol. 473, no. 2, pp. 2
[grid_creator id = ‘5’]



Very helpful
Lovely just what I was searching for. Thanks to the author for taking his time on this one.
I am not sure where you’re getting your info, but good topic. I needs to spend some time learning much more or understanding more. Thanks for great information I was looking for this info for my mission.
Enough knowledge extracted from this article, thanks
Pretty cool knowledge
Good for me, thanks.
This excellent website really has all of the info I needed concerning this subject and didn’t know who to ask.
You helped me in my studies, so I thank you.
Thanks for the time
Your writing skill is commendable
This blog site is very good! How can I make one like this !
Hey There. I found your blog using bing. This is a very well written article. I will be sure to bookmark it and come back to read more of your usefull information. Thanks for the post.mortgage banker new york
Good one, and detailed too.